Uma bala de ouro para o câncer:as nanopartículas fornecem uma versão direcionada da terapia fototérmica para o câncer
 p A cor de uma suspensão de nanocages depende da espessura das paredes das gaiolas e do tamanho dos poros dessas paredes. Como sua cor, sua capacidade de absorver luz e convertê-la em calor pode ser controlada com precisão. Crédito:WUSTL
p A cor de uma suspensão de nanocages depende da espessura das paredes das gaiolas e do tamanho dos poros dessas paredes. Como sua cor, sua capacidade de absorver luz e convertê-la em calor pode ser controlada com precisão. Crédito:WUSTL
p Em uma palestra que ele proferiu em 1906, o médico alemão Paul Ehrlich cunhou o termo Zuberkugel, ou "bala mágica, "como uma abreviatura para um tratamento médico altamente direcionado. p Balas mágicas, também chamadas de balas de prata, por causa da crença folclórica de que apenas balas de prata podem matar criaturas sobrenaturais, continuam a ser o objetivo dos esforços de desenvolvimento de medicamentos hoje.
p Uma equipe de cientistas da Universidade de Washington em St. Louis está atualmente trabalhando em uma solução mágica para o câncer, uma doença cujos tratamentos são notoriamente indiscriminados e inespecíficos. Mas suas balas são de ouro em vez de prata. Literalmente.
p As balas de ouro são nanocages de ouro que, quando injetado, se acumulam seletivamente nos tumores. Quando os tumores são posteriormente banhados por luz laser, o tecido circundante mal é aquecido, mas os nanocages convertem luz em calor, matando as células malignas.
p Em um artigo recém-publicado na revista
Pequena , a equipe descreve o tratamento fototérmico bem-sucedido de tumores em camundongos.
p A equipe inclui Younan Xia, Ph.D., o professor James M. McKelvey de Engenharia Biomédica na Escola de Engenharia e Ciências Aplicadas, Michael J. Welch, Ph.D., professor de radiologia e biologia do desenvolvimento na Faculdade de Medicina, Jingyi Chen, Ph.D., professor assistente de pesquisa de engenharia biomédica e Charles Glaus, Ph.D., um associado de pesquisa de pós-doutorado no Departamento de Radiologia.
p "Vimos mudanças significativas no metabolismo e na histologia do tumor, "diz Welch, "o que é notável, visto que o trabalho foi exploratório, a 'dose' do laser não foi maximizada, e os tumores eram direcionados 'passivamente' em vez de 'ativamente'. "
 p Nanocages de ouro (à direita) são caixas ocas feitas pela precipitação de ouro em nanocubos de prata (à esquerda). A prata simultaneamente se desgasta de dentro do cubo, entrar na solução através dos poros que se abrem nos cantos recortados do cubo. Crédito:WUSTL
p Nanocages de ouro (à direita) são caixas ocas feitas pela precipitação de ouro em nanocubos de prata (à esquerda). A prata simultaneamente se desgasta de dentro do cubo, entrar na solução através dos poros que se abrem nos cantos recortados do cubo. Crédito:WUSTL
p
Por que os nanocages esquentam
p Os próprios nanocages são inofensivos. "Sais de ouro e colóides de ouro têm sido usados para tratar a artrite por mais de 100 anos, "diz Welch." As pessoas sabem o que o ouro faz no corpo e é inerte, portanto, esperamos que seja uma abordagem não tóxica. "
p "A chave para a terapia fototérmica, "diz Xia, "é a capacidade das gaiolas de absorver luz com eficiência e convertê-la em calor."
p Suspensões dos nanocages de ouro, que têm aproximadamente o mesmo tamanho de uma partícula de vírus, nem sempre são amarelos, como seria de esperar, mas, em vez disso, pode ser de qualquer cor do arco-íris.
p Eles são coloridos por algo chamado ressonância de plasmon de superfície. Alguns dos elétrons no ouro não estão ancorados em átomos individuais, mas, em vez disso, formam um gás de elétron flutuante, Xia explica. A luz incidindo sobre esses elétrons pode levá-los a oscilar como um só. Esta oscilação coletiva, o plasmon de superfície, escolhe um comprimento de onda particular, ou cor, fora da luz incidente, e isso determina a cor que vemos.
p Os artesãos medievais faziam vitrais vermelho-rubi misturando cloreto de ouro em vidro fundido, um processo que deixou minúsculas partículas de ouro suspensas no vidro, says Xia.
p The resonance — and the color — can be tuned over a wide range of wavelengths by altering the thickness of the cages' walls. For biomedical applications, Xia's lab tunes the cages to 800 nanometers, a wavelength that falls in a window of tissue transparency that lies between 750 and 900 nanometers, in the near-infrared part of the spectrum.
p Light in this sweet spot can penetrate as deep as several inches in the body (either from the skin or the interior of the gastrointestinal tract or other organ systems).
p The conversion of light to heat arises from the same physical effect as the color. The resonance has two parts. At the resonant frequency, light is typically both scattered off the cages and absorbed by them.
p By controlling the cages' size, Xia's lab tailors them to achieve maximum absorption.
p
Passive targeting
p "If we put bare nanoparticles into your body, " says Xia, "proteins would deposit on the particles, and they would be captured by the immune system and dragged out of the bloodstream into the liver or spleen."
p To prevent this, the lab coated the nanocages with a layer of PEG, a nontoxic chemical most people have encountered in the form of the laxatives GoLyTELY or MiraLAX. PEG resists the adsorption of proteins, in effect disguising the nanoparticles so that the immune system cannot recognize them.
p Instead of being swept from the bloodstream, the disguised particles circulate long enough to accumulate in tumors.
p A growing tumor must develop its own blood supply to prevent its core from being starved of oxygen and nutrients. But tumor vessels are as aberrant as tumor cells. They have irregular diameters and abnormal branching patterns, but most importantly, they have thin, leaky walls.
p The cells that line a tumor's blood vessel, normally packed so tightly they form a waterproof barrier, are disorganized and irregularly shaped, and there are gaps between them.
p The nanocages infiltrate through those gaps efficiently enough that they turn the surface of the normally pinkish tumor black.
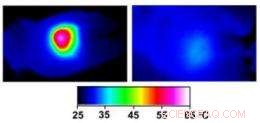 p Infrared images made while tumors were irradiated with a laser show that in nanocage-injected mice (left), the surface of the tumor quickly became hot enough to kill cells. In buffer-injected mice (right), the temperature barely budged. This specificity is what makes photothermal therapy so attractive as a cancer therapy. Credit:WUSTL
p Infrared images made while tumors were irradiated with a laser show that in nanocage-injected mice (left), the surface of the tumor quickly became hot enough to kill cells. In buffer-injected mice (right), the temperature barely budged. This specificity is what makes photothermal therapy so attractive as a cancer therapy. Credit:WUSTL
p
A trial run
p In Welch's lab, mice bearing tumors on both flanks were randomly divided into two groups. The mice in one group were injected with the PEG-coated nanocages and those in the other with buffer solution. Several days later the right tumor of each animal was exposed to a diode laser for 10 minutes.
p The team employed several different noninvasive imaging techniques to follow the effects of the therapy. (Welch is head of the oncologic imaging research program at the Siteman Cancer Center of Washington University School of Medicine and Barnes-Jewish Hospital and has worked on imaging agents and techniques for many years.)
p During irradiation, thermal images of the mice were made with an infrared camera. As is true of cells in other animals that automatically regulate their body temperature, mouse cells function optimally only if the mouse's body temperature remains between 36.5 and 37.5 degrees Celsius (98 to 101 degrees Fahrenheit).
p At temperatures above 42 degrees Celsius (107 degrees Fahrenheit) the cells begin to die as the proteins whose proper functioning maintains them begin to unfold.
p In the nanocage-injected mice, the skin surface temperature increased rapidly from 32 degrees Celsius to 54 degrees C (129 degrees F).
p In the buffer-injected mice, Contudo, the surface temperature remained below 37 degrees Celsius (98.6 degrees Fahrenheit).
p To see what effect this heating had on the tumors, the mice were injected with a radioactive tracer incorporated in a molecule similar to glucose, the main energy source in the body. Positron emission and computerized tomography (PET and CT) scans were used to record the concentration of the glucose lookalike in body tissues; the higher the glucose uptake, the greater the metabolic activity.
p The tumors of nanocage-injected mice were significantly fainter on the PET scans than those of buffer-injected mice, indicating that many tumor cells were no longer functioning.
p The tumors in the nanocage-treated mice were later found to have marked histological signs of cellular damage.
p
Active targeting
p The scientists have just received a five-year, $2, 129, 873 grant from the National Cancer Institute to continue their work with photothermal therapy.
p Despite their results, Xia is dissatisfied with passive targeting. Although the tumors took up enough gold nanocages to give them a black cast, only 6 percent of the injected particles accumulated at the tumor site.
p Xia would like that number to be closer to 40 percent so that fewer particles would have to be injected. He plans to attach tailor-made ligands to the nanocages that recognize and lock onto receptors on the surface of the tumor cells.
p In addition to designing nanocages that actively target the tumor cells, the team is considering loading the hollow particles with a cancer-fighting drug, so that the tumor would be attacked on two fronts.
p But the important achievement, from the point of view of cancer patients, is that any nanocage treatment would be narrowly targeted and thus avoid the side effects patients dread.
p The TV and radio character the Lone Ranger used only silver bullets, allegedly to remind himself that life was precious and not to be lightly thrown away. If he still rode today, he might consider swapping silver for gold.


